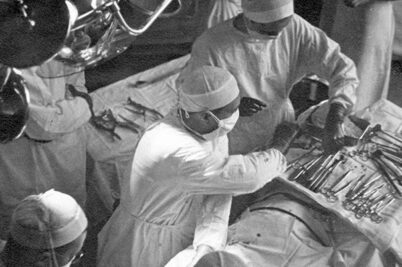
UW surgery turns 100
The first day of classes must have felt like a leap of faith. It was 1924 and just three faculty members charged with teaching three medical students made up the new surgery academic department in the University of Wisconsin’s new School of Medicine. At the Wisconsin General Hospital, community surgeons were called in to help teach the students how to set bones, treat sepsis, alleviate inflammation and perform minor surgery.