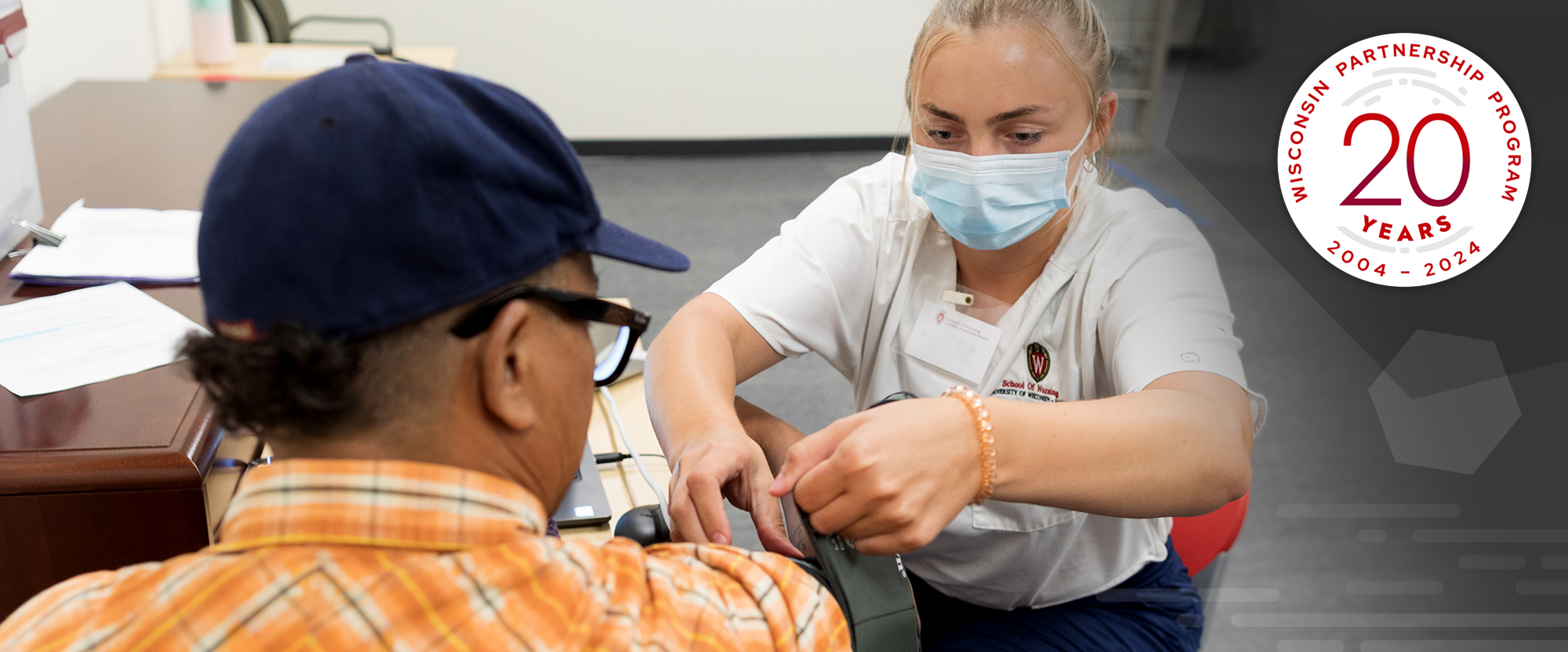
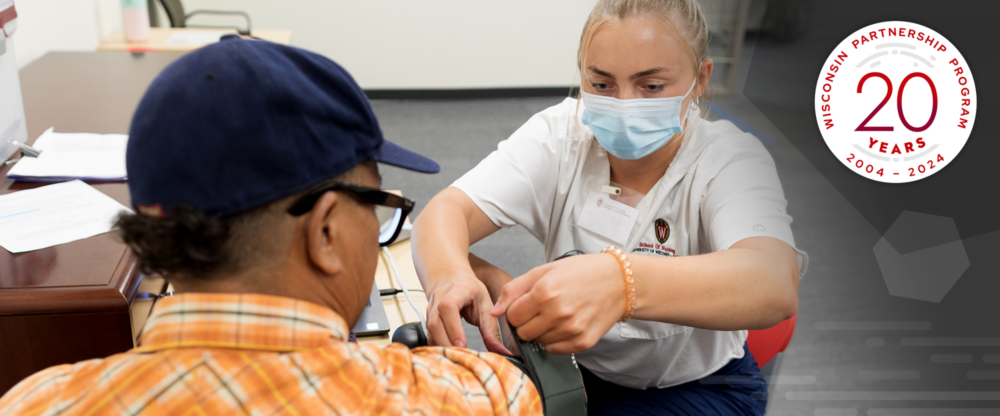
Wisconsin Partnership Program announces Community Impact Grant funding opportunity
The Wisconsin Partnership Program (WPP) at the University of Wisconsin School of Medicine and Public Health has released the first of two 2024 Community Impact Grant Program Requests for Partnerships.