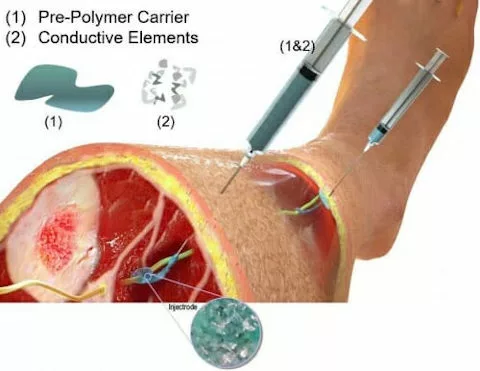
Today’s neuromodulation treatments rely on surgically implanted devices that can cost up to six figures, require complex procedures to install, and often fail — given that they’re rigid devices attempting to mesh with soft biological tissue.
The researchers’ system leverages an entirely new way of thinking.
“You can inject the liquid around the nerve, and it cures in the body to create a wired contact,” says Kip Ludwig, a UW–Madison professor of biomedical engineering and neurological surgery. “Typical implants are really stiff, and so as the body moves, they wear and tear and break down. Our liquid cures, and the result is much closer to the normal elasticity of tissue. You can actually stretch it and increase its size 150 percent to 200 percent without losing its conductivity.”
To create the injectrode, the researchers mixed a silicone base — similar to surgical glue — with small metal particles to make the liquid sufficiently conductive.
They put their device through a battery of Food and Drug Administration preclinical tests, and used it to induce heart rate changes in pigs by stimulating their vagus nerve, an approach that’s shown promise for treating heart failure, hypertension and other maladies.
“We essentially went through the standard repertoire of electrochemical tests to show this acts like a standard wire electrode that could be used to stimulate the nerve,” says James Trevathan, a postdoctoral fellow in Ludwig’s lab and first author on the study.