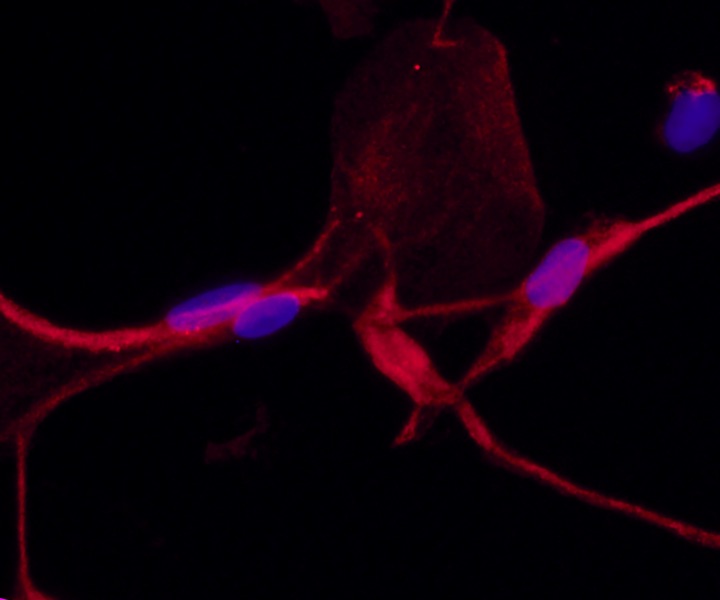
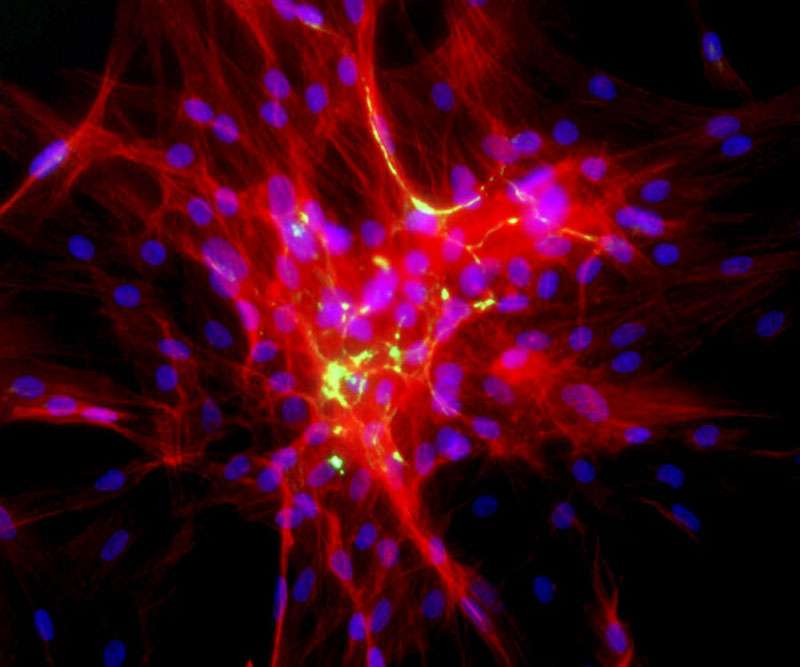
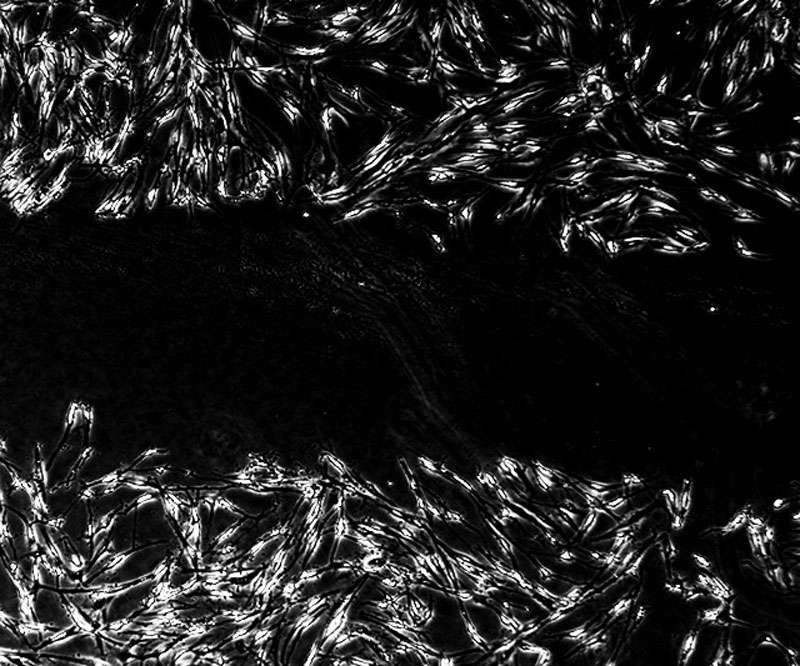
Welham’s group was already studying the role fibrocytes play in wound healing. Fibrocytes are seen as “bad actors” that lead to scarring. In the monocyte family of blood cells, they arise from the bone marrow and are usually found in low numbers in the bloodstream. But when the wound healing process begins, fibrocytes rush to the site of the wound. Some transform into the mesenchymal cells that make up connective tissue. Left unchecked, these cells can promote fibrosis, or scarring.
Until recently, the process by which fibrocytes differentiate from monocytes was “a black box and poorly understood,’’ Welham says. “We wondered if we could shed some light on the differentiation process and, additionally, use the resulting mesenchymal cells for a therapeutic purpose. Could these cells have a good side?”
One part of the study described how the fibrocytes go through a 14-day process of transforming from a round monocyte cell to an elongated mesenchymal cell. Next, they used proteomics to identify the proteins associated with this transformation. They showed that differentiation involves a complete retooling of cell metabolism, including greater dependence on a pathway called oxidative phosphorylation. They then compared the cells to other mesenchymal cell types, including cells obtained from skin, vocal fold, and bone marrow, finding that at the proteome level, they are distinct, although fibrocytes do have some mesenchymal features.
Next, they tried using the mature fibrocytes to build multilayered tissues. They placed the cells within a scaffold to form a base layer, put epithelial cells on top, and found that the layer built from fibrocytes was able to support the maturation of a functional epithelium. They were less successful when they tried to also make the epithelium using blood-derived fibrocytes.