“Given this massive investment of resources, viral RNA genome replication is arguably one of the most important processes in infection, and it is already a major target for virus control,” Ahlquist says.
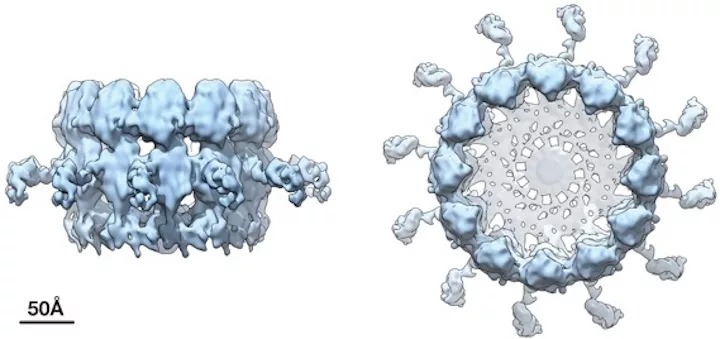
The team’s prior work also showed that the crown structure, which sits atop the spherule neck, contains a key viral protein that creates the replication complex and copies the viral RNA.
The new higher-resolution cryo-EM images and complementary results show that the crown is composed of 12 copies of the key viral RNA replication protein, arranged like staves in a barrel.
It also provides the team with clues as to how it works. The replication protein is large and contains segments, or domains, that perform different functions. Two of these domains — RNA polymerase and RNA capping domains— are common across numerous positive-strand RNA viruses and contribute to synthesizing new viral genome copies.
Using an approach that involved labeling part of the replication complex with gold nanoparticles, Ahlquist’s team discerned the orientation of these domains within the crown structure. How these domains are physically organized is one of the most important issues for understanding how the replication complex functions.
For instance, they found that the polymerase domain that actually synthesizes new RNA is positioned at the apex of the crown, providing clues about the early steps in the replication process in which the starting viral RNA template is recruited into the complex and the spherule is formed. It also provides context for later steps, during which the template is copied to make progeny genomes for packaging into new infectious virus particles.
“Such advances will reveal in increasing detail how these complexes assemble and operate, and thus how they might be best attacked,” Ahlquist explains. “These insights should provide the basis for novel, stronger antiviral mechanisms.”
The results also provide a strong foundation for further experiments to define the replication complex structure and function at even higher levels, the researchers say.
“We hope to continue to improve the RNA replication complex crown structure to provide additional important refinements in the future,” Ahlquist says. “We also hope to address growing indications from our work that conformational changes in these proteins are critical to their multiple functions.”